Easy® SARS-CoV-2 WE – 192 test
The “Easy® SARS-CoV-2″ kit is a unique and comprehensive One Step Real-Time RT-PCR in vitro diagnostic test for the qualitative detection of Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) and for the screening of today’s most commonly represented variants.
A quick, easy and complete response for COVID-19 screening
- Starting material: RNA extracted from nasopharyngeal or oropharyngeal swabs, sputum and broncho-alveolar washes (BAL).
- Format: each kit contains sufficient reagents to perform 192 complete tests, including qualitative detection of SARS-CoV-2 and variant screening. Each kit includes the reagents required to perform RT-PCR, complete of positive and negative controls.
- Workflow high processivity flexibility and CE IVD: the system allows to run from 1 to 94 samples in parallel in the same run in less than 2 hours
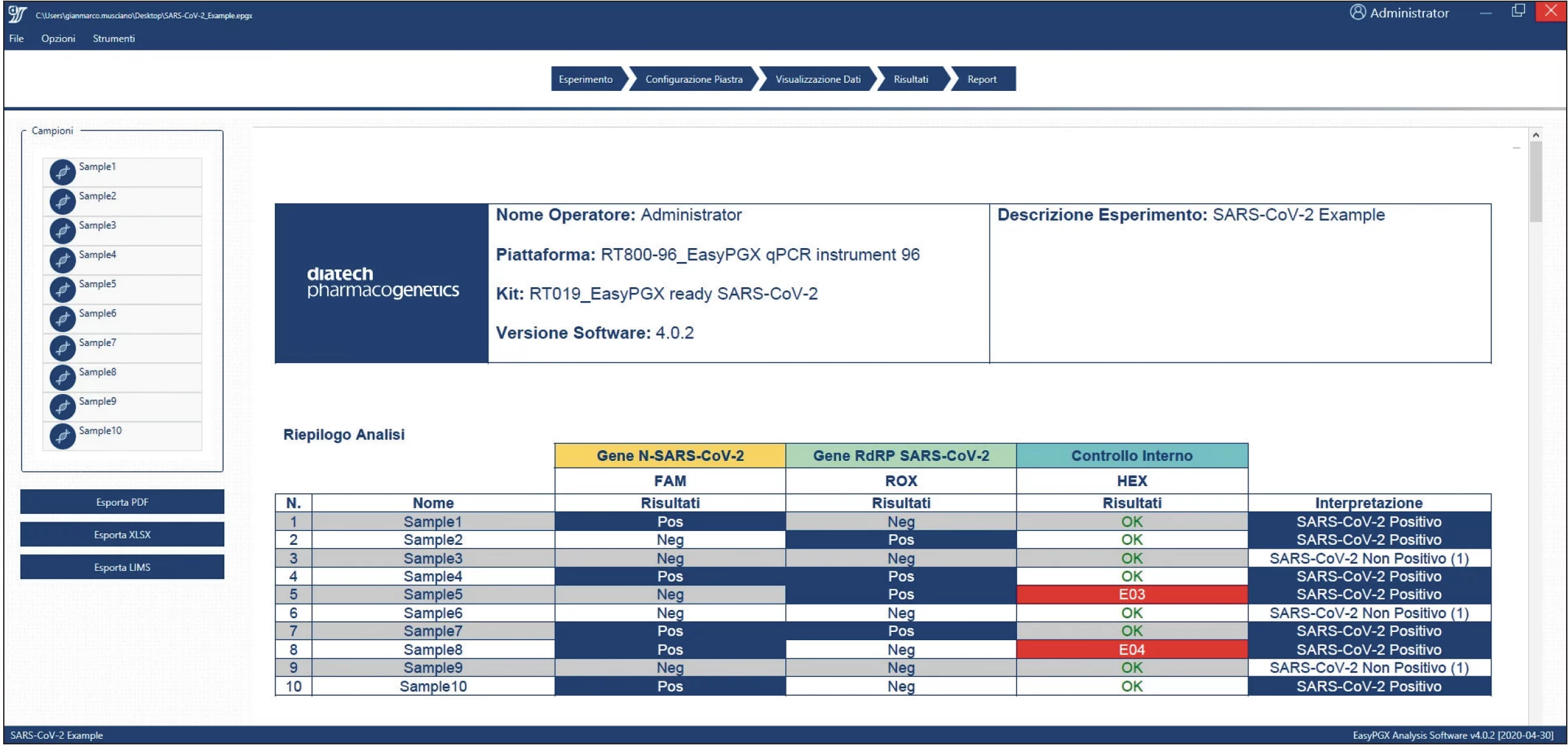
- Fully automatic data processing with EasyPGX® Analysis Software.
EasyPGX® Analysis Software: the CE-IVD solution for data processing fully automatic
- Developed with “wizard” to facilitate the definition, analysis and exportation of results.
- Unique solution and platform for interactive visualization and comparison of raw data and processed results.
- Possibility to insert patient ID with a barcode reader.
- Possibility to read and track codes and batches of reagents.
- Compatible with Laboratory Information Management Systems (LIMS).
- Reports can be customized and exported in various formats (pdf, excel).
Enter your information below to download the brochure
Category
Realtime PCR
Validated tools
EasyPGX qPCR instrument 96 (Diatech Pharmacogenetics)
CFX96 Real-Time PCR Detection System (Bio-Rad)
ABI 7500/7500 Fast (ThermoFisher)
QIAGEN Rotor-Gene Q
