Last summer, the European Society of Medical Oncology (ESMO) published recommendations on how to use next-generation sequencing in clinical practice.
The ESMO recommendations, included in the publication: „Recommendations for the use of next-generation sequencing (NGS) for patients with metastatic cancers: a report from the ESMO Precision Medicine Working Group”, are based on the analysis of clinical validity and economic sustainability related to the introduction of next generation sequencing (NGS) in clinical practice at 3 different levels: from a public health perspective, from the perspective of academic clinical research institutions and taking into consideration potential advantages or disadvantages for each individual patient. The review considers the eight cancer types with the highest number of deaths around the world and defines the clinical utility of various genetic markers with the ESCAT scale (ESMO Clinical Scale for Actionability of molecular Targets). The ESCAT scale classifies druggable genomic variants in a rank of four levels:
- ESCAT I: genomic alterations with predictive value validated in clinical trials.
- ESCAT II: genomic alterations associated with drugs response in phase I / II studies or in retrospective analyses of randomized studies.
- ESCAT III: genomic alterations validated in another cancer, but not in the specific one to be treated.
- ESCAT IV: genomic hypothetically targetable alterations based of preclinical data.
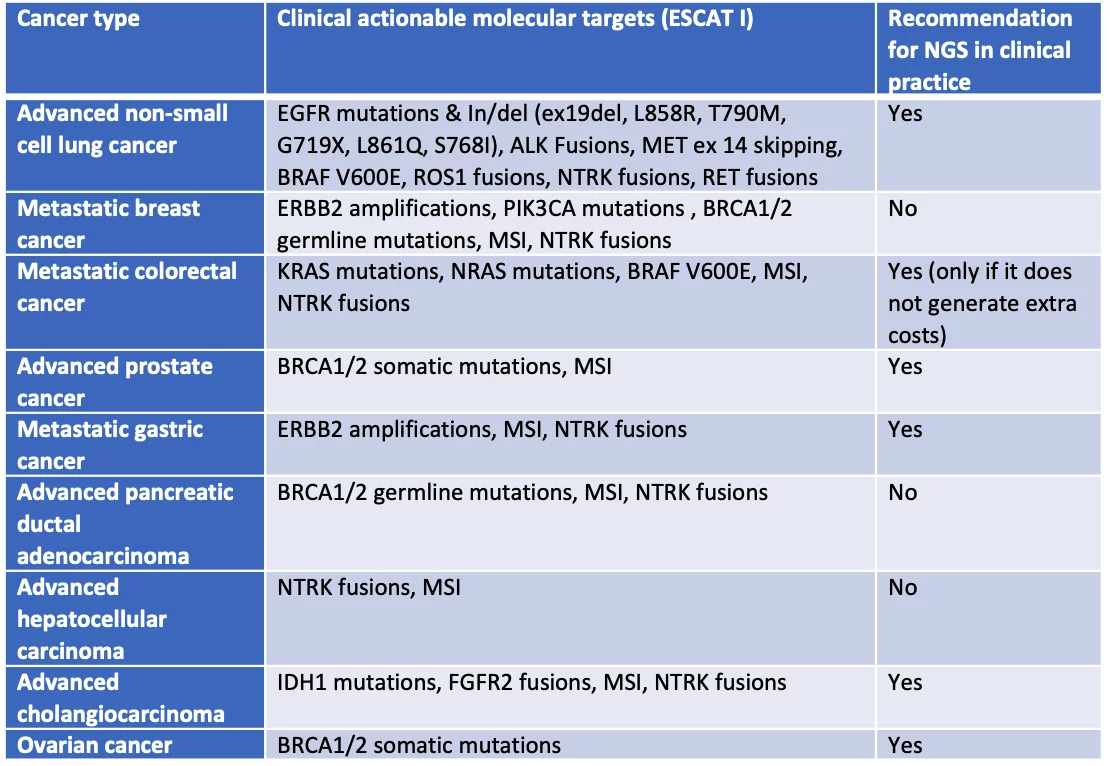
The use of multigene next generation sequencing in daily clinical practice is recommended for the following diseases: non-small cell lung cancer (NSCLC), prostate cancer, ovarian cancer and cholangiocarcinoma. Considering these cancer types, the introduction of NGS technology guarantees clinical benefits and economic sustainability compared to alternative methods, while it remains a possible option in case of colorectal cancer, if it does not generate extra costs compared, for example, to Real Time PCR.
Large panels of genes should be used only if they generate an acceptable increase in the overall cost, drugs included. This is based on evidence that the research of ESCAT II-III-IV variants does not seem to contribute to the identification of a significantly higher number of responder patients and therefore, should be considered only for research purposes or for possible inclusion in approved clinical trials.
The following table summarizes the recommendations and ESCAT I variants for each cancer type taken into consideration:
A separate discussion needs to be considered for the evaluation of Tumor Mutational Burden (TMB) to select patients suitable for therapy with anti-PD (L) 1 antibodies. Based on the results of the KN158 study, ESMO recommends to determine TMB only in cases of cervical cancer, neuroendocrine cancers, salivary cancers, vulvar cancer, and thyroid cancers.
Diatech Pharmacogenetics offers a complete, ready-to-use and CE-IVD validated application portfolio based on NGS and Real Time PCR assays. Visit www.diatechpharmacogenetics.com for more information.
References
- Mosele F, Remon J, Mateo J, Westphalen CB, Barlesi F, Lolkema MP, Normanno N, Scarpa A, Robson M, Meric-Bernstam F, Wagle N, Stenzinger A, Bonastre J, Bayle A, Michiels S, Bièche I, Rouleau E, Jezdic S, Douillard JY, Reis-Filho JS, Dienstmann R, André F. Recommendations for the use of next-generation sequencing (NGS) for patients with metastatic cancers: a report from the ESMO Precision Medicine Working Group. Ann Oncol. 2020 Nov;31(11):1491-1505. doi: 10.1016/j.annonc.2020.07.014. Epub 2020 Aug 24. PMID: 32853681.

