EasyPGX qPCR instrument 96
EasyPGX ready EGFR plus (cod. RT030) è l’unico kit completo per uso in vitro diagnostico che permette il rilevamento delle mutazioni T790M e C797S (due varianti) dell’oncogene EGFR mediante Real Time PCR. La mutazione T790M rappresenta sia il principale meccanismo di resistenza acquisita nei confronti degli inibitori della tirosin-chinasi di EGFR di prima e seconda generazione, sia il target degli EGFR TKI di terza generazione. Mentre la mutazione C797S è stata riconosciuta da numerosi studi1 come uno dei fattori responsabili della resistenza verso questi ultimi farmaci (maggiori informazioni2).
Linea EasyPGX: dal tessuto al risultato in meno di 3 ore
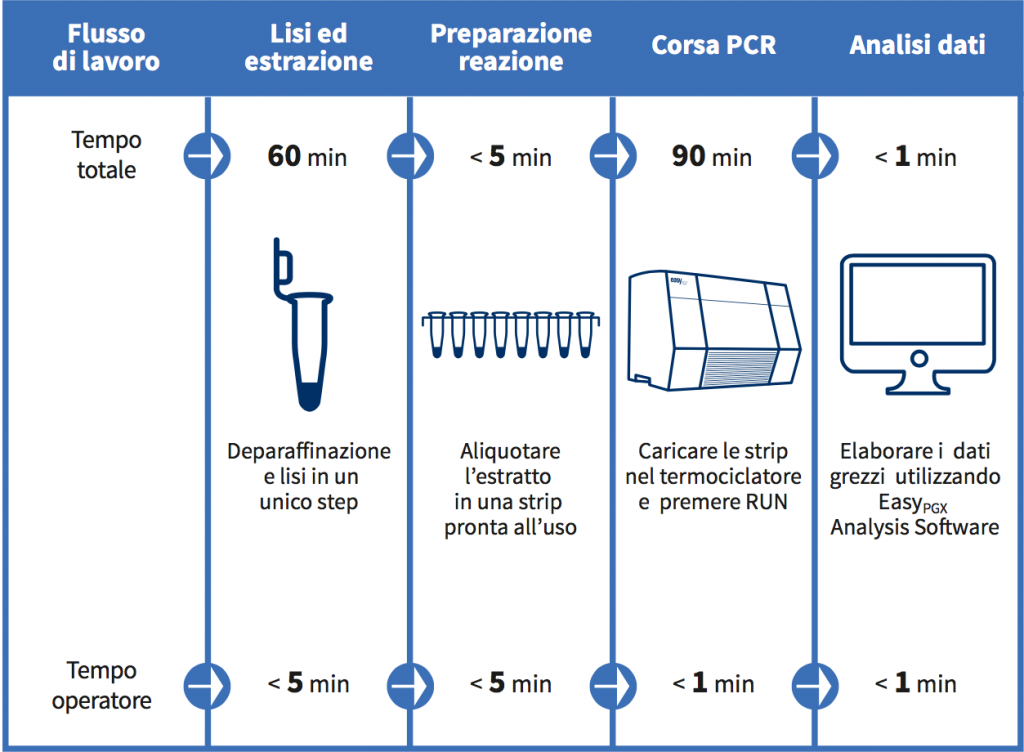
Caratteristiche principali del prodotto
Materiale di partenza: tessuto tumorale (fresco, congelato, FFPE), plasma.
Principio di funzionamento del kit: Real Time PCR per rilevazione selettiva di DNA mutato.
Completo di tutti i reagenti: estrazione da tessuto inclusa nel kit.
Pronto all’uso: tutti i reagenti di amplificazione necessari, completi di mastermix, sono pre-aliquotati in strip pronte all’uso. Le strip possono essere conservate a temperatura ambiente.
Semplice da utilizzare: nessuna necessità di congelamento o scongelamento; gli step di pipettamento sono ridotti al minimo e così il rischio di errori o contaminazione.
Certificazione: marcatura CE IVD dei reagenti e del sistema EasyPGX completo di strumenti, accessori e software di analisi automatica.
Linea completa: va ad aggiungersi alla linea di farmacogenetica oncologica EasyPGX ready to yoUSE.
1 Letteratura EasyPGX ready EGFR plus (cod. RT030) Letteratura EasyPGX ready EGFR plus (cod. RT030)
- Yu HA et al., Acquired Resistance of EGFR-Mutant Lung Cancer to a T790M-Specific EGFR Inhibitor: Emergence of a Third Mutation (C797S) in the EGFR Tyrosine Kinase Domain. JAMA Oncol. 1:982–984 (2015).
- Soria et al., Osimertinib in Untreated EGFR-Mutated Advanced Non–Small-Cell Lung Cancer. New Engl J Med Jan 11;378(2):113-125 (2017) .
- Niederst et al., The allelic context of the C797S mutation acquired upon treatment with third generation EGFR inhibitors impacts sensitivity to subsequent treatment strategies. Clin. Cancer Res. 21, 3924–3933 (2015).
2 Maggiori informazioni
In FAM tre saggi per il rilevamento delle mutazioni ed un saggio di controllo per la valutazione del contenuto (qualitativo e quantitativo) di DNA nel campione:
- EGFR C797S c.2389: il saggio rileva la mutazione C797S (c.2389 T>A)
- EGFR C797S c.2390: il saggio rileva la mutazione C797S (c.2390 G>C)
- EGFR T790M: il saggio rileva la mutazione T790M – mutazione driver che dovrebbe essere già presente nel campione
- EGFR ctrl: il saggio rileva una regione del gene EGFR priva di qualsiasi polimorfismo/mutazione ad oggi conosciuto Ogni pozzetto amplifica in HEX controllo interno endogeno su tutte e 4 le mix per verificare la corretta esecuzione della procedura di amplificazione e l’eventuale presenza di inibitori
In HEX un controllo interno endogeno viene amplificato in tutte e 4 le mix per verificare che la procedura sia stata eseguita correttamente e per escludere l’eventuale presenza di inibitori.